PRISM
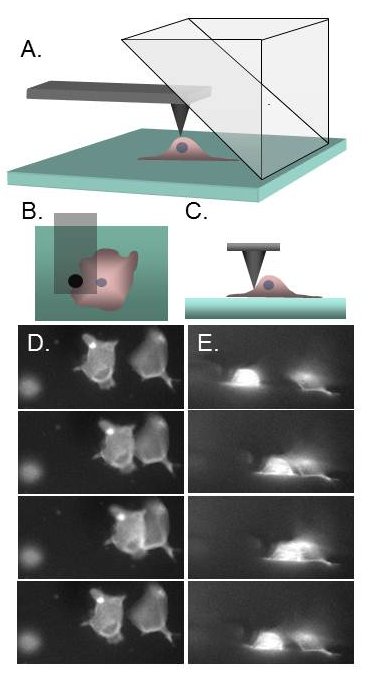
Recent advances in biophysical and biomechanical tools, nanotechnology and computing have increased our ability to probe the mechanical properties of cells and tissue. Mechanical loading of cells is an area of intense interest due to downstream effects such as signaling, gene expression, differentiation and motility, to name a few. However, structural information during applied stress is limited to our ability to image the cells under load. The traditional “plan-view” epi-fluorescence imaging is limiting because the force (and hence dominant strains) are often in the z-direction. While this can be ameliorated by 3D confocal microscopy, image stack collection can be slow in comparison with loading and cell response, and resolution in the axial direction compromised by the axial point spread function. We are developing a video rate, high resolution imaging of the cells under load in both the horizontal and vertical imaging planes simultaneously. This is accomplished by arranging a 90º reflecting optic above the specimen within the field of view of the standard epi-fluorescence imaging system. We call this method PRISM for Pathway Rotated Imaging for Sideways Microscopy. We will integrate PRISM with two force techniques, magnetic tweezers and atomic force microscopy, and begin application to cell and nuclear mechanics.