Thrombosis Cluster
CISMM has a long and productive history of collaboration with investigators studying the mechanical processes related to blot clotting (1-9).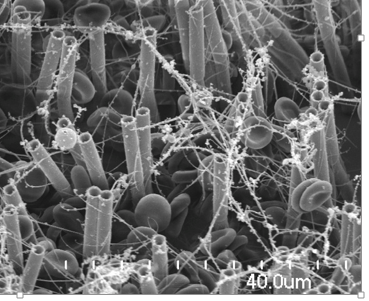
Clotting Diagnostics: ASAP. We have developed a novel technique for measuring the time dependent elastic properties of microliter quantities of biofluids using an array of magnetically actuated surface attached posts (ASAP). Our first target application is blood clot diagnostics. The image to the right is an SEM micrograph of a blood clot formed on our ASAP posts.
Permeability of Blood Clots. Although traditional high-throughput (HT) clotting time analysis has high predictive power for bleeding diatheses, it is not able to predict thrombosis risk. Because permeability relates more directly to the biophysical aspects of pathological clotting, we hypothesize that it will reveal physiologically relevant differences in clot properties that are not observable using traditional HT clotting time assays. Using microbead tracking methods on Panoptes, we will collect clot permeability, and optically derived clotting times for each specimen loaded onto a 96- or 384-well plate, with correlated high resolution clot structural data collected on a separate imaging system.
Mechanics of Fibrin Molecules, Fibers, and Networks. The mechanical properties of blood clots are of central importance to normal hemostasis. A normal clot is sufficiently strong to seal the site of vessel injury and prevent blood loss from a circulatory system under pressure. Using atomic force microscopy and other force methods, we investigate the mechanical properties of individual fibrin molecules, single fibers and small fiber networks to elucidate the underlying molecular origins of fibrin mechanical properties.
References:
1. Spero, R.C., R.K. Sircar, R. Schubert, R.M. Taylor, 2nd, A.S. Wolberg, and R. Superfine, Nanoparticle diffusion measures bulk clot permeability. Biophys J, 2011. 101(4): p. 943-50.
2. Hudson, N.E., J.R. Houser, E.T. O’Brien, 3rd, R.M. Taylor, 2nd, R. Superfine, S.T. Lord, and M.R. Falvo, Stiffening of individual fibrin fibers equitably distributes strain and strengthens networks. Biophys J, 2010. 98(8): p. 1632-40.
3. Houser, J.R., N.E. Hudson, L. Ping, E.T. O’Brien, 3rd, R. Superfine, S.T. Lord, and M.R. Falvo, Evidence that alphaC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J, 2010. 99(9): p. 3038-47.
4. Falvo, M.R., O.V. Gorkun, and S.T. Lord, The molecular origins of the mechanical properties of fibrin. Biophys Chem, 2010. 152(1-3): p. 15-20.
5. Campbell, R.A., M. Aleman, L.D. Gray, M.R. Falvo, and A.S. Wolberg, Flow profoundly influences fibrin network structure: implications for fibrin formation and clot stability in haemostasis. Thromb Haemost, 2010. 104(6): p. 1281-4.
6. O’Brien, E.T., 3rd, M.R. Falvo, D. Millard, B. Eastwood, R.M. Taylor, 2nd, and R. Superfine, Ultrathin self-assembled fibrin sheets. Proc Natl Acad Sci U S A, 2008. 105(49): p. 19438-43.
7. Falvo, M.R., D. Millard, E.T. O’Brien, 3rd, R. Superfine, and S.T. Lord, Length of tandem repeats in fibrin’s alphaC region correlates with fiber extensibility. J Thromb Haemost, 2008. 6(11): p. 1991-3.
8. Guthold, M., W. Liu, E.A. Sparks, L.M. Jawerth, L. Peng, M. Falvo, R. Superfine, R.R. Hantgan, and S.T. Lord, A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys, 2007. 49(3): p. 165-81.
9. Liu, W., L.M. Jawerth, E.A. Sparks, M.R. Falvo, R.R. Hantgan, R. Superfine, S.T. Lord, and M. Guthold, Fibrin fibers have extraordinary extensibility and elasticity. Science, 2006. 313(5787): p. 634.
1. Spero, R.C., R.K. Sircar, R. Schubert, R.M. Taylor, 2nd, A.S. Wolberg, and R. Superfine, Nanoparticle diffusion measures bulk clot permeability. Biophys J, 2011. 101(4): p. 943-50.
2. Hudson, N.E., J.R. Houser, E.T. O’Brien, 3rd, R.M. Taylor, 2nd, R. Superfine, S.T. Lord, and M.R. Falvo, Stiffening of individual fibrin fibers equitably distributes strain and strengthens networks. Biophys J, 2010. 98(8): p. 1632-40.
3. Houser, J.R., N.E. Hudson, L. Ping, E.T. O’Brien, 3rd, R. Superfine, S.T. Lord, and M.R. Falvo, Evidence that alphaC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J, 2010. 99(9): p. 3038-47.
4. Falvo, M.R., O.V. Gorkun, and S.T. Lord, The molecular origins of the mechanical properties of fibrin. Biophys Chem, 2010. 152(1-3): p. 15-20.
5. Campbell, R.A., M. Aleman, L.D. Gray, M.R. Falvo, and A.S. Wolberg, Flow profoundly influences fibrin network structure: implications for fibrin formation and clot stability in haemostasis. Thromb Haemost, 2010. 104(6): p. 1281-4.
6. O’Brien, E.T., 3rd, M.R. Falvo, D. Millard, B. Eastwood, R.M. Taylor, 2nd, and R. Superfine, Ultrathin self-assembled fibrin sheets. Proc Natl Acad Sci U S A, 2008. 105(49): p. 19438-43.
7. Falvo, M.R., D. Millard, E.T. O’Brien, 3rd, R. Superfine, and S.T. Lord, Length of tandem repeats in fibrin’s alphaC region correlates with fiber extensibility. J Thromb Haemost, 2008. 6(11): p. 1991-3.
8. Guthold, M., W. Liu, E.A. Sparks, L.M. Jawerth, L. Peng, M. Falvo, R. Superfine, R.R. Hantgan, and S.T. Lord, A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys, 2007. 49(3): p. 165-81.
9. Liu, W., L.M. Jawerth, E.A. Sparks, M.R. Falvo, R.R. Hantgan, R. Superfine, S.T. Lord, and M. Guthold, Fibrin fibers have extraordinary extensibility and elasticity. Science, 2006. 313(5787): p. 634.