Cell Mechanics Cluster
Cells are force exerting and force sensing objects. Our collaborators are studying cell motility in cancer, cell force response in endothelia, and mechanical properties as related to vesicle formation. In these cases we have developed new tools in our 3DFM magnetics system, and responded to our collaborators in engineering permanent magnetic systems to use for population studies of gene expression and phosphorylation in response to applied force. Here we feature recent results from Keith Burridge (UNC) studying mechanical properties of nuclei, Ellie Tzima (UNC) investigating the effect of forces on the recruitment of PE-CAM at the plasma membrane, Nancy Kleckner (Harvard) for studying genes under compression and Nenad Bursac (Duke University) applying our new Panoptes system for the study of cardiac myocytes.
Manipulation of endogenous kinase activity in living cells using photoswitchable inhibitory peptides
ACS Synth Biol. 2014 Nov 21;3(11):788-95. doi: 10.1021/sb5001356.
Optogenetic control of endogenous signaling can be an important tool for probing cell behavior. Using the photoresponse of the LOV2 domain of Avena sativa phototropin 1, we developed analogues of kinase inhibitors whose activity is light dependent. Inhibitory peptides were appended to the Jα helix, where they potently inhibited kinases in the light but were sterically blocked from kinase interaction in the dark. Photoactivatable inhibitors for cyclic-AMP dependent kinase (PKA) and myosin light chain kinase (MLCK) are described, together with studies that shed light on proper positioning of the peptides in the LOV domain. These inhibitors altered endogenous signaling in living cells and produced light-dependent changes in cell morphodynamics.
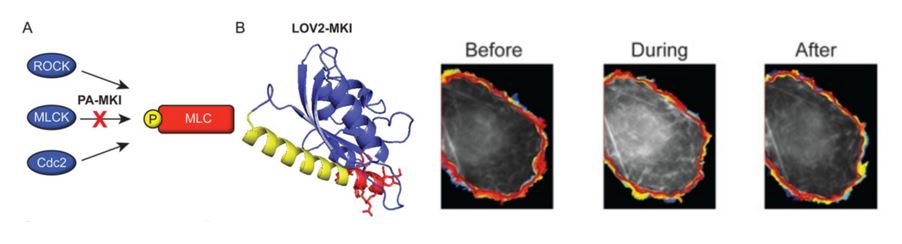
Left: Construction of a photoactivatable myosin light chain kinase inhibitor (PA-MKI). (A) Schematic showing kinases that phosphorylate regulatory myosin light chain. (B) I-TASSER prediction of the conformation for PA-MKI. Right: Changes in the protrusion dynamics of COS-7 cells in response to PA-MKI activation. Snapshot of raw image data before (left), during (center), and after (right) light stimulation of a cell expressing PA-MKI. Colors represent the cell edge over the entire imaging interval (blue, early time points; red, late time points).
The Guanine-Nucleotide Exchange Factor SGEF Plays a Crucial Role in the Formation of Atherosclerosis
Samson T, van Buul, J., Droon, J., Welch, C., Bakker, E., Matlung, H., van den Berg, T., Sharek, L., Doerschuk, C., Hahn, K., & Burridge, K. PloS one. 8(1):55202. (2013) PMC3555862. PDF
The passage of leukocytes across the endothelium and into arterial walls is a critical step in the development of atherosclerosis. To explore the in vivo role of this protein during inflammation, we generated SGEF-deficient mice. When crossed with ApoE null mice and fed a Western diet, mice lacking SGEF showed a significant decrease in the formation of atherosclerosis in multiple aortic areas. A fluorescent biosensor revealed local activation of RhoG around bead-clustered ICAM-1 in mouse aortic endothelial cells. Notably, this activation was decreased in cells from SGEF-deficient aortas compared to wild type. In addition, scanning electron microscopy of intimal surfaces of SGEF2/2 mouse aortas revealed reduced docking structures around beads that were coated with ICAM-1 antibody. Similarly, under conditions of flow, these beads adhered less stably to the luminal surface of carotid arteries from SGEF2/2 mice. Taken together, these results show for the first time that a Rho-GEF, namely SGEF, contributes to the formation of atherosclerosis by promoting endothelial docking structures and thereby retention of leukocytes at athero-prone sites of inflammation experiencing high shear flow. SGEF may therefore provide a novel therapeutic target for inhibiting the development of atherosclerosis.
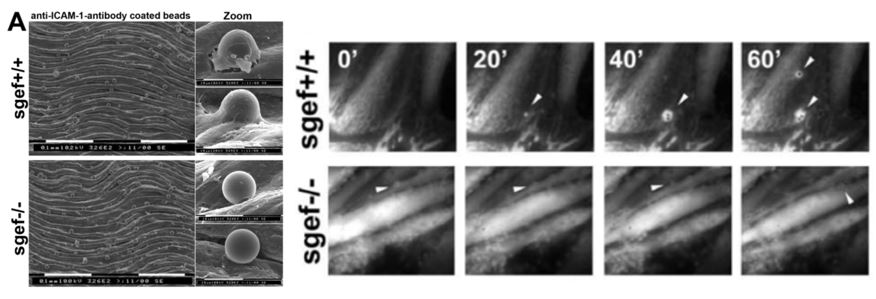
A: Isolated aortas from SGEF+/+ and SGEF2/2 mice were mounted with the intimal site facing up. After TNFa treatment, the aortas were overlaid with anti-ICAM-1-Ab coated beads for 2 hours, before unbound beads were washed off and imaged by scanning electron microscopy. Representative scanning electron microscopy show attached anti-ICAM-1-Ab coated beads to the intimal surface of aortas from SGEF+/+ and SGEF2/2 animals. Right panels show magnification of adherent bead. Scale bars represent 0.1 um or 10 um, as indicated. B. Representative time-lapse imaging of ELMO-YFP recruitment to sites of adhesion of anti-ICAM-1-Ab coated beads on TNF-a-treated MAECs from SGEF+/+ or SGEF2/2 animals. Arrowheads show bead location and only in the wildtype cells, ELMO recruitment is observed.
Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus.
Nat. Cell Biol. 2014;(August 2013). doi:10.1038/ncb2927.
Christophe Guilluy, Lukas D. Osborne, Laurianne Van Landeghem, Lisa Sharek, Richard Superfine,
Rafael Garcia-Mata and Keith Burridge
Mechanical forces influence many aspects of cell behaviour. Forces are detected and transduced into biochemical signals by force-bearing molecular elements located at the cell surface, in adhesion complexes or in cytoskeletal structures1. The nucleus is physically connected to the cell surface through the cytoskeleton and the linker of nucleoskeleton and cytoskeleton (LINC) complex, allowing rapid mechanical stress transmission from adhesions to the nucleus2. Although it has been demonstrated that nuclei experience force3, the direct effect of force on the nucleus is not known. Here we show that isolated nuclei are able to respond to force by adjusting their stiffness to resist the applied tension. Using magnetic tweezers, we found that applying force on nesprin-1 triggers nuclear stiffening that does not involve chromatin or nuclear actin, but requires an intact nuclear lamina and emerin, a protein of the inner nuclear membrane. Emerin becomes tyrosine phosphorylated in response to force and mediates the nuclear mechanical response to tension. Our results demonstrate that mechanotransduction is not restricted to cell surface receptors and adhesions but can occur in the nucleus.
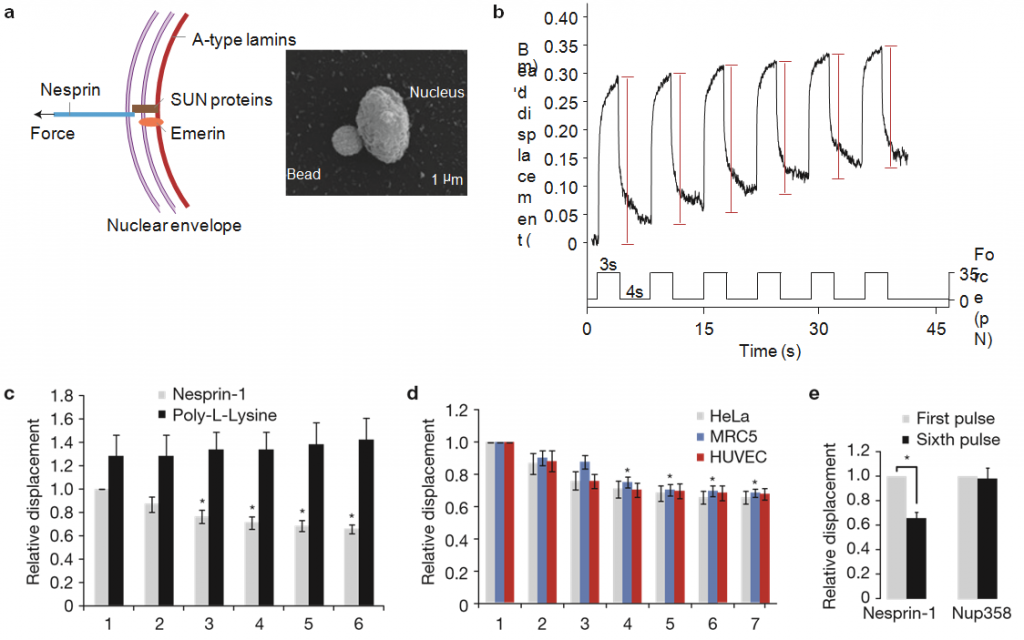
Figure 1 Isolated nuclei stiffen in response to force applied on nesprin-1. (a) Left, diagram of the LINC complex showing where tensional forces were applied to mimic the transmission of mechanical stress from the cytoskeleton to the nucleus. Right, scanning electron micrograph of a magnetic bead attached to a nucleus isolated from a HeLa cell. Result is representative from 6 independent experiments. (b) Typical displacement of a 2.8µm bead coated with anti-nesprin-1 antibody bound to an isolated nucleus during force pulse application. Stiffening is indicated by decreased displacement during later pulses. (c) Change in bead displacement during 6 force pulses applied to beads coated with anti-nesprin-1 antibody (n= 18 beads) or poly-L-lysine (n=14 beads) and bound to nuclei isolated from HeLa cells. Displacements were calculated relative to the first pulse of force applied to beads coated with anti-nesprin-1 (error bars represent s.e.m., *P<0.05, data were collected from 3 independent experiments and analysed by one-way analysis of variance (ANOVA)). (d) Change in bead displacement during 7 force pulses applied to beads coated with anti-nesprin-1 bound to nuclei isolated from HeLa cells (n= 18 beads), MRC5 cells (n= 21 beads) or HUVECs (n=15 beads). Displacements were calculated relative to the first pulse of force (error bars represent s.e.m., *P <0.05, data were collected from 3 independent experiments and analysed by one-way ANOVA). (e) Change in bead displacement between the first and sixth pulse of force applied to beads coated with anti-nesprin-1 antibody (n=18 beads) or antiNUP358 antibody (n=16 beads) and bound to nuclei isolated from HeLa cells. Displacements were calculated relative to the first pulse of force (error bars represent s.e.m., *P <0.05, data were collected from 3 independent experiments and analysed by a two-tailed unpaired t-test).
Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells.
Nature Communications 2014. doi:10.1038/ncomms4984.
Caitlin Collins, Lukas D. Osborne, Christophe Guilluy, Zhongming Chen, E. Tim O’Brien III, John S. Reader, Keith Burridge, Richard Superfine & Ellie Tzima
Endothelial cells (ECs) lining blood vessels express many mechanosensors, including platelet endothelial cell adhesion molecule-1 (PECAM-1), that convert mechanical force into biochemical signals. While it is accepted that mechanical stresses and the mechanical properties of ECs regulate vessel health, the relationship between force and biological response remains elusive. Here we show that ECs integrate mechanical forces and extracellular matrix (ECM) cues to modulate their own mechanical properties. We demonstrate that the ECM influences EC response to tension on PECAM-1. ECs adherent on collagen display divergent stiffening and focal adhesion growth compared with ECs on fibronectin. This is because of protein kinase A (PKA)-dependent serine phosphorylation and inactivation of RhoA. PKA signalling regulates focal adhesion dynamics and EC compliance in response to shear stress in vitro and in vivo. Our study identifies an ECM-specific, mechanosensitive signalling pathway that regulates EC compliance and may serve as an atheroprotective mechanism that maintains blood vessel integrity in vivo.
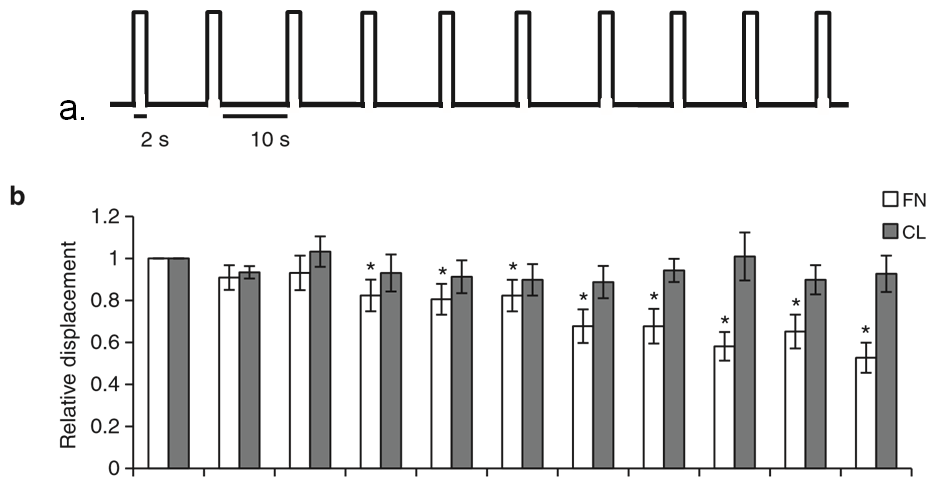
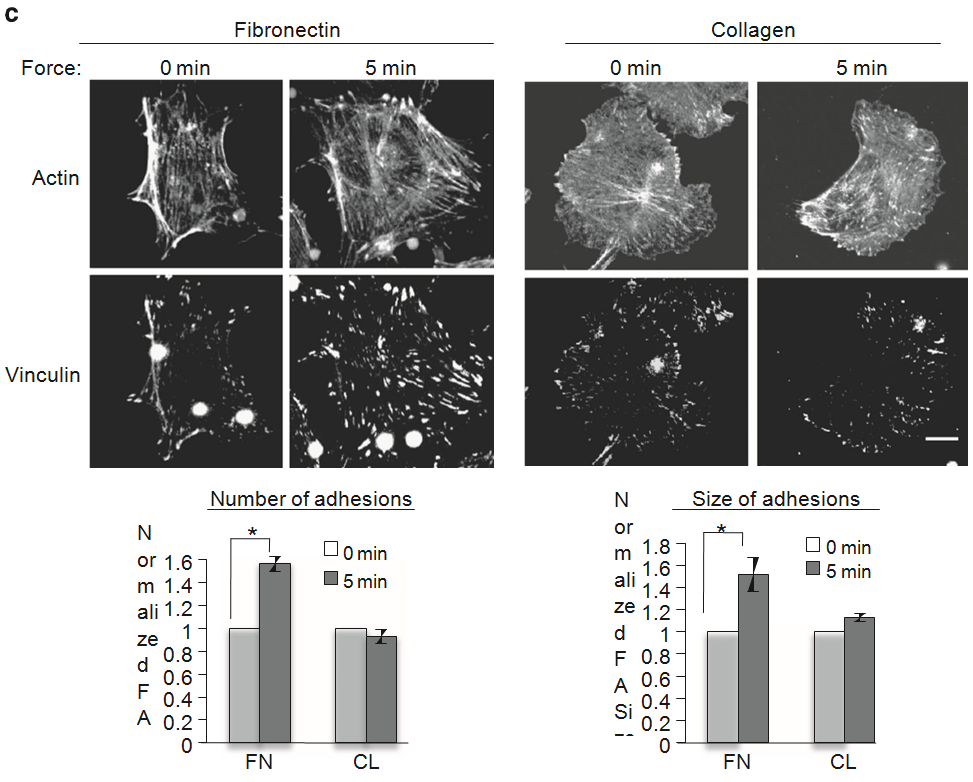
Figure 1 | ECM composition determines the cellular response to tension on PECAM-1. (a) Pulsatile force regimen applied with magnetic tweezers, consisting of a 2-s, 100-pN pulse of force, followed by a 10-s period of rest, repeated over a 2-min time course. (b) Average relative anti-PECAM-1-coated bead displacements induced by pulsatile force regimen. ECs were seeded on FN or CL 4 h before incubation with magnetic beads. Average displacements were calculated relative to the first pulse of force. (n430 beads per condition from three independent experiments). Error bars represent s.e.m., *Po0.05 using a Student’s t-test. (c) Adherent ECs on FN or CL were incubated with anti-PECAM-1-coated magnetic beads and subjected to force with a permanent magnet for the indicated times. ECs were fixed-stained with phalloidin and an anti-vinculin antibody to mark focal adhesions. Focal adhesion number and size were quantified using the NIH ImageJ software. Values were normalized to the FN ‘No Force’ condition. n430 cells per condition from three independent experiments, *Po0.05 using a Student’s t-test, scale bar ¼ 10 mm.
Magnetic force micropiston: an integrated force/microfluidic device for the application of compressive forces in a confined environment.
Rev. Sci. Instrum. 2014;85(2):023704. doi:10.1063/1.4864085.
Fisher JK, Kleckner N.
Cellular biology takes place inside confining spaces. For example, bacteria grow in crevices, red blood cells squeeze through capillaries, and chromosomes replicate inside the nucleus. Frequently, the extent of this confinement varies. Bacteria grow longer and divide, red blood cells move through smaller and smaller passages as they travel to capillary beds, and replication doubles the amount of DNA inside the nucleus. This increase in confinement, either due to a decrease in the available space or an increase in the amount of material contained in a constant volume, has the potential to squeeze and stress objects in ways that may lead to changes in morphology, dynamics, and ultimately biological function. Here, we describe a device developed to probe the interplay between confinement and the mechanical properties of cells and cellular structures, and forces that arise due to changes in a structure’s state. In this system, the manipulation of a magnetic bead exerts a compressive force upon a target contained in the confining space of a microfluidic channel. This magnetic force microfluidic piston is constructed in such a way that we can measure (a) target compliance and changes in compliance as induced by changes in buffer, extract, or biochemical composition, (b) target expansion force generated by changes in the same parameters, and (c) the effects of compression stress on a target’s structure and function. Beyond these issues, our system has general applicability to a variety of questions requiring the combination of mechanical forces, confinement, and optical imaging.
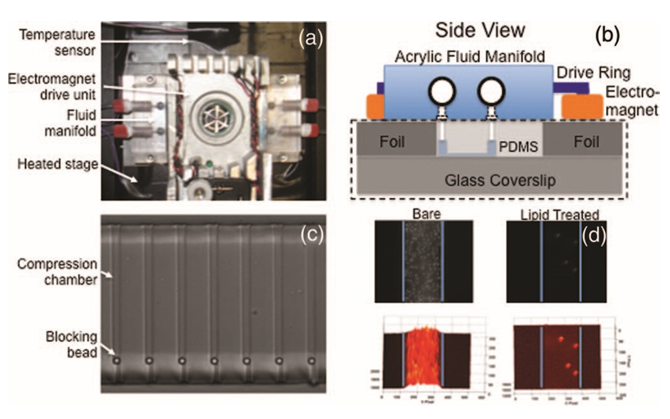
FIG. 5. (a) The complete micropiston system, with the electromagnet drive unit in the center flanked by acrylic fluid delivery manifolds. These three components sit atop a heated plate (surface covered with black insulation) inserted into the 96–well plate adapter of a motorized stage (Proscan II, Prior Scientific, Rockland, MA). (b) Illustrated side view of the micropiston chip (dashed black rectangle), fluid manifold, and electromagnets. (c) Multiple compression chambers (small channels) of the micropiston system loaded with 4.5 μm blocking beads. (d) Non–specific adsorption to the inner channel surfaces is reduced significantly using a lipid treatment. The top two images are unprocessed fluorescence, bottom two have fluorescent intensity mapped to surface height. Blue lines indicate channel walls. The adsorption of 200 nm polystyrene beads to channel surfaces after a 2 h incubation is shown for a bare channel (left) and treated channel (right).
Myocyte Cells
This video was taken with our Panoptes optical system, and shows a monolayer of Sprague-Dawley neonatal rat cardiomyocyte cells that have been plated on a flexible polyacrylamide substrate. The contractions we see are initiated by a square wave of electric current delivered to the edge of the monolayer. The wave of contraction is passed from cell to cell, very similarly to the way it is passed within an intact heart. Embedded within the flexible substrates are fluorescent microspheres, and these are used as markers of the deformation of the sheet. Our experiments are first geared toward seeing how the stiffness of the flexible substrate alters the amplitude of contractions, and from there calculating forces within the sheet. We will then extend the technique to high throughput analysis of the effects of drugs and toxins, on these forces and on the patterns of force transmission.