nanoManipulator
 The nanoManipulator System integrates haptic force feedback control of a nanoscale probe tip with imaging in a fluorescence optical microscope. It has been used for virus, chromosome, cell and clot manipulation experiments. Beyond developments in instrumentation and software, CISMM develops methodologies for force measurements. Through our work with our Thrombosis collaborators on fibrin fiber mechanics, we have developed methods of fabricating “structured surfaces” (SS). These engineered surfaces enable stretching experiments in which biological fiber samples are suspended across channels and there for free of the confounding mechanical influence underlying surfaces. We will be developing integrated in-plane uniaxial and biaxial strain capabilities that will greatly expand the host of mechanical interrogations our collaborators can perform.
The nanoManipulator System integrates haptic force feedback control of a nanoscale probe tip with imaging in a fluorescence optical microscope. It has been used for virus, chromosome, cell and clot manipulation experiments. Beyond developments in instrumentation and software, CISMM develops methodologies for force measurements. Through our work with our Thrombosis collaborators on fibrin fiber mechanics, we have developed methods of fabricating “structured surfaces” (SS). These engineered surfaces enable stretching experiments in which biological fiber samples are suspended across channels and there for free of the confounding mechanical influence underlying surfaces. We will be developing integrated in-plane uniaxial and biaxial strain capabilities that will greatly expand the host of mechanical interrogations our collaborators can perform.
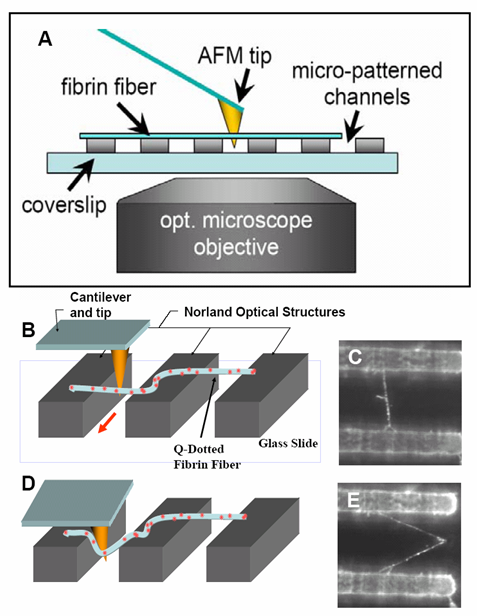
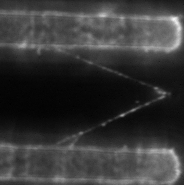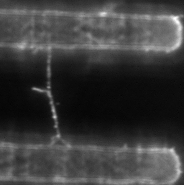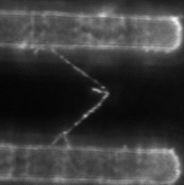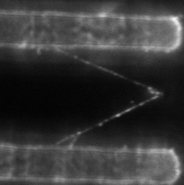
At right is (A) side view of experimental set up. Inverted optical microscope objective images the fibrin manipulation through the coverslip with structured channel-ridge surface. Suspended fibrin fibers are labeled with fluorescent beads and then stretched with the afm tip. Perspective view (b,d) of afm tip, and fibrin fiber suspended across channel. Fluorescence images of suspended fiber before being stretched (c) after and stretched to ~200% strain by the afm tip (e).
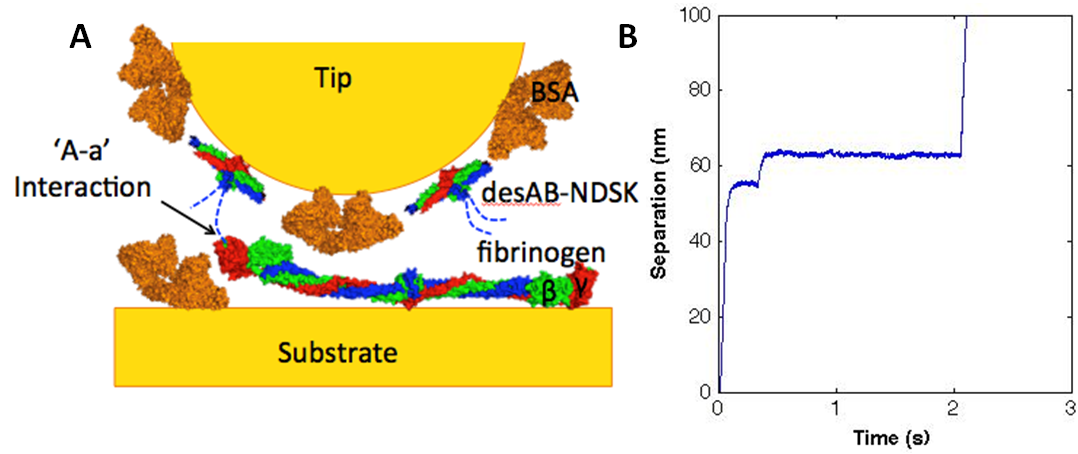
Constant force unfolding of single molecule fibrinogen through the ‘A-a’ knob-hole interaction
Fibrin, the polymerized form of the soluble plasma protein fibrinogen, plays a critical role in hemostasis as the structural scaffold of blood clots. The primary functions of fibrin are to withstand the shear forces of blood flow and provide mechanical stability to the clot, protecting the wound. As a viscoelastic material, fibrin networks possess unique mechanical properties critical to its physiological function. Studies show that fibrin clots are extensible and elastic.
Fibrin fibers are the most highly extensible biological polymers, with strains of up to five fold before breaking. How the fibrin fibers accommodate such high strain is not completely understood but studies show that extensibility of fibrin fiber is due, at least in part, to unfolding of individual fibrinogen monomers. As a multifunctional tool capable of force spectroscopy, the atomic force microscope (AFM) has enabled examination of single molecule protein-protein interactions. Using the AFM to preform constant force pulling experiments, extension-time traces show the unfolding of fibrin molecules. These force curves provide mechanical information on the probability of unfolding and forced rupture of the ‘A-a’ knob-hole interaction. Direct measurements of the stability of the interaction and the kinetics of unfolding the fibrin molecule as a function of force can be obtained and applied to understanding full fiber extensibility.
Characterizing the Mechanical Properties of Fibrin Sheets
Fibrin is a fibrous protein which polymerizes into networks to form the structural framework for blood clots. Experiments have previously been done to study the mechanical properties of single fibrin fibers, showing breaking strains of 300%, reversibility, and elasticity.
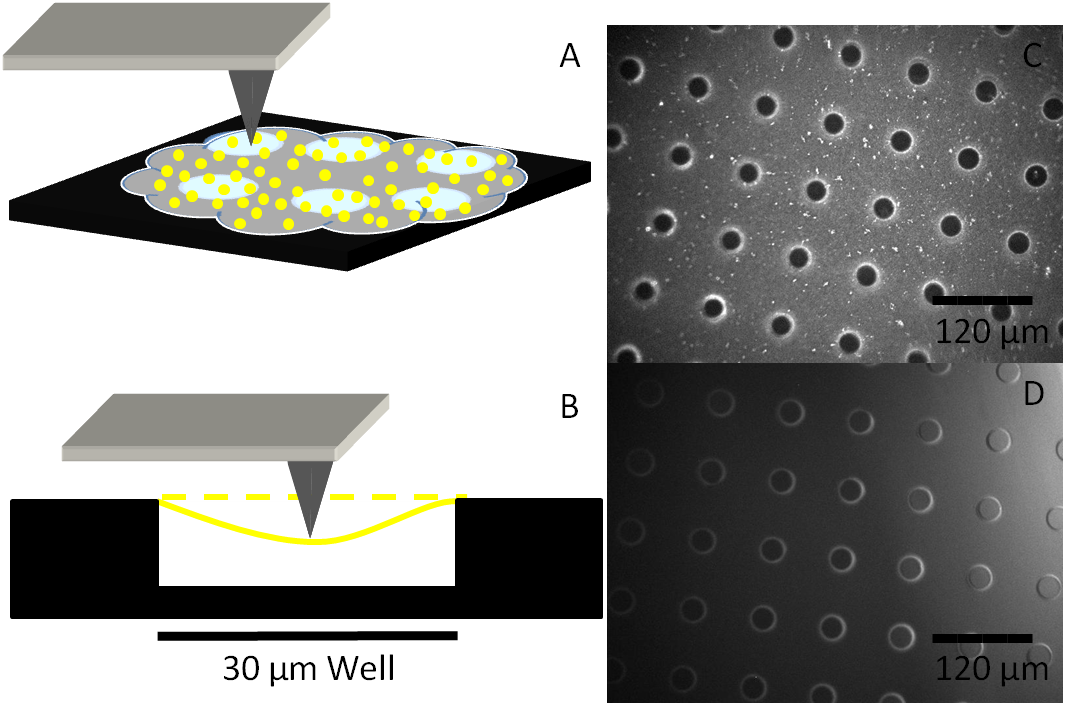
Fibrin has also been shown to form sheets which are flat, elastic, and mechanically continuous. The goal for this research is to be able to characterize and describe the amount of force required to break a fibrin sheet and find the young’s modulus for these sheets. To this date, fibrin sheets have been able to be consistently polymerized over micro-patterned wells and have been imaged. Further research with the Asylum MFP-3D atomic force microscope will give data pertaining to the force required to cause failures of interactions within a sheet.
The Mechanics of Fibrinolysis
The fibrinolytic system (FS) regulates the dissolution of fibrin clots and is mediated by the protein plasmin which binds to fibrin, cleaving the molecule in three different locations: αC domain, Bβ1-42, and then across the middle of the coiled coils. A well-regulated FS is medically important due to its potential to cause harm, if unregulated, by producing embolisms, heart attacks, and strokes. The goal of this project is to study the effects of the mechanical and physical properties of fibrin on the FS at the scale of single fibers as opposed to whole clots.
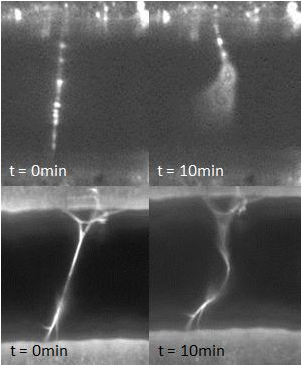
Relevant fibrin properties include the diameter and tension of fibrin fibers as well as the concentrations of proteins such as plasmin and thrombin. High concentrations of thrombin have been shown to yield thinner fibers and vice versa; this result will be confirmed and fibrin diameters will be measured with an SEM. Moreover, the effect of fibrin tension on the FS (i.e. whether fibers under higher levels of tension are more or less susceptible to fibrinolysis when exposed to plasmin) will be investigated using a 3DFM. To date, we have observed the FS system to fail by causing the fibrin fibers to elongate instead of fully lysing. This result may superficially explain why some fibrin clots do not readily dissolve. Furthermore, this phenomenon is not dependent on the concentration of plasmin: the percentage of elongated vs. lysed fibers remained roughly the same for plasmin concentrations ranging from 0.06 U/mL to 6 U/mL. On the other hand, fibrin elongation appears to depend on thrombin concentration: fibers that polymerized by lower thrombin concentrations showed higher rates of elongation than fibers formed under higher concentrations of thrombin.
1. Hudson, N.E., J.R. Houser, E.T. O’Brien, 3rd, R.M. Taylor, 2nd, R. Superfine, S.T. Lord, and M.R. Falvo, Stiffening of individual fibrin fibers equitably distributes strain and strengthens networks. Biophys J, 2010. 98(8): p. 1632-40.
2. Houser, J.R., N.E. Hudson, L. Ping, E.T. O’Brien, 3rd, R. Superfine, S.T. Lord, and M.R. Falvo, Evidence that alphaC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J, 2010. 99(9): p. 3038-47.
3. Falvo, M.R., O.V. Gorkun, and S.T. Lord, The molecular origins of the mechanical properties of fibrin. Biophys Chem, 2010. 152(1-3): p. 15-20.
4. O’Brien, E.T., 3rd, M.R. Falvo, D. Millard, B. Eastwood, R.M. Taylor, 2nd, and R. Superfine, Ultrathin self-assembled fibrin sheets. Proc Natl Acad Sci U S A, 2008. 105(49): p. 19438-43.
5. Falvo, M.R., D. Millard, E.T. O’Brien, 3rd, R. Superfine, and S.T. Lord, Length of tandem repeats in fibrin’s alphaC region correlates with fiber extensibility. J Thromb Haemost, 2008. 6(11): p. 1991-3.
6. Guthold, M., W. Liu, E.A. Sparks, L.M. Jawerth, L. Peng, M. Falvo, R. Superfine, R.R. Hantgan, and S.T. Lord, A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys, 2007. 49(3): p. 165-81.
7. Falvo, M.R., E.T. O’Brien, L. Ping, L.A. Hartle, S.T. Lord, and R. Superfine, Mechanical evaluation of individual fibrin fibers with Atomic Force Microscopy. Biophysical Journal, 2007: p. 523a-523a.
8. Liu, W., L.M. Jawerth, E.A. Sparks, M.R. Falvo, R.R. Hantgan, R. Superfine, S.T. Lord, and M. Guthold, Fibrin fibers have extraordinary extensibility and elasticity. Science, 2006. 313(5787): p. 634.
9. Guthold, M., W. Liu, B. Stephens, S.T. Lord, R.R. Hantgan, D.A. Erie, R.M. Taylor, Jr., and R. Superfine, Visualization and mechanical manipulations of individual fibrin fibers suggest that fiber cross section has fractal dimension 1.3. Biophys J, 2004. 87(6): p. 4226-36.
10. Falvo, M.R., R.M. Taylor II, A. Helser, V. Chi, F.P. Brooks Jr. , S. Washburn, and R. Superfine, Nanometre-scale rolling and sliding of carbon nanotubes. Nature, 1999. 397(21 January): p. 236-238.
11. Taylor, R., G. Matthews, A. Negishi, M. Guthold, M. Falvo, R. Superfine, S. Washburn, and F. Brooks, Molecular structure investigation and modification using the nanoManipulator. Journal of Molecular Graphics & Modelling, 1998. 16(4-6): p. 291-292.
12. Falvo, M.R., S. Washburn, R. Superfine, M. Finch, F.P. Brooks, V. Chi, and R.M. Taylor, Manipulation of individual viruses: Friction and mechanical properties. Biophysical Journal, 1997. 72(3): p. 1396-1403.
13. Falvo, M.R., G.J. Clary, R.M. Taylor, V. Chi, F.P. Brooks, S. Washburn, and R. Superfine, Bending and buckling of carbon nanotubes under large strain. Nature, 1997. 389(6651): p. 582-584.